Insecticidal Activities of Melaleuca leucadendron Leaf Essential Oil Against Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae) and Tribolium castaneum (Herbst) (Coleoptera:Tenebrionidae)
Introduction
Insect pests cause heavy losses of stored grain quantitatively and qualitatively throughout the world (Madrid et al., 1990). Insect damage in stored grains may amount to 10% - 40% in countries where modern storage technologies have not been introduced (Shaaya et al., 1997). Insect pests cause the loss of stored food grain in tropical and semitropical environments (Tripathi et al., 2001). Chemical control of insects in storage has been used for a long time, but has serious drawbacks (Sharaby, 1988). Careless and indiscriminate use of synthetic pesticides has led to a number of wellknown problems. Some of these are contamination of food, soil, ground water, rivers, lakes, oceans, air etc. with toxic residues, side effects on non-target insects and other organisms, increase of the number of pest species resistant to pesticides, and pest resurgence. In addition, many non-lethal as well as lethal accidents occurred due to mishandling of highly toxic synthetic products.
As a logical consequence of the undesirable side effects of these products, there is a growing awareness in industrialized and also in developing countries, of the toxicological and environmental problems involved in the use of synthetic pesticides. This awareness has led to a steadily increasing movement towards a more environmental – oriented, sustainable agriculture with low or no input of toxic synthetic pesticides and other agricultural chemicals in an attempt to preserve and protect the environment as well as human health.
The plant kingdom is by far the most efficient “factory” of compounds. It synthesizes secondary metabolic products which can partly be considered as weapons to defend plants against pests and diseases which have competed with them since time immemorial.
Historically, chemicals derived from plants have been an important source of insecticides. The insecticidal properties of the alkaloid nicotine, for example, have been known since 1800s (Metcalf et al., 1962; Ware, 1986). Botanical materials such as pyrethrum, rotenone, and nicotine were among the first compounds used to control agricultural insect pests (Perkins, 1985; Grainge and Ahmed, 1988). With the advent of effective and economical synthetic insecticides, however, plant-based insecticides used in agriculture were displaced. As technological (resistance, resurgence and secondary outbreaks) and cultural (health and environmental) problems involved in the use of synthetic pesticides have increased, interest in naturally occurring plant compounds as alternative sources of pest control has been renewed (Perkins, 1985). Currently, novel, as well as established, secondary plant compounds is being evaluated as alternatives for crop protection in many countries.
Several commercial produced plant-derived compounds have exhibited good potential as insecticides. Azadirachtin, a triterpenoid from the neem tree, Azadirachta indica A. Juss (Meliaceae), has growth-regulating and antifeedent properties against numerous insect pests (Warthen, 1979; Rembold, 1988). Commercial preparations of many household insecticides are augmented with pyrethrins, a complex of esters extracted from flowers of Chrysanthemum cinerariefolium (Treviranus) (Compositae) (Bell et al., 1990). Alkaloids that have been used commercially include ryanodine, the active ingredient in ryania (Ryania spp., Flacourtiaeae) the principal components extracted from the seeds of sabadilla, Schoenocaulon spp., Liliaceae (Ware 1986; Bell et al., 1990). Although too expensive for commercial synthesis, rotenone, an isoflavonoid procured primarily from two genera of tropical legumes, several Derris and Lonchocarpus spp., (Fabaceae), has been moderately effective against insects with chewing mouthparts (Bell et al., 1990).
Plants are rich sources of natural substances that can be utilized in the development of environmentally safe methods for insect control. The deleterious effects of certain purified phytochemicals or crude plant extracts on insects are manifested in several ways including toxicity (Hiremath et al., 1997), growth retardation (Breuer and Schmidt, 1995), feeding inhibition (Klepzig and Schlyter, 1999; Wheeler and Isman, 2000), oviposition deterrence (Dimock and Renwick, 1991; Hermawan et al., 1994; Zhao et al., 1998), suppression of calling behavior (Khan and Saxena, 1986) and reduction of fecundity and fertility (El-Ibrashy,1974; Muthukrishnan and Pushpalatha, 2001). Although a wide variety of effects provide potential alternatives for the use of synthetic chemical insecticides, certain plant families, particularly Meliaceae, Asteraceae, Rutaceae, Labiaceae, Annonaceae and Canellaceae are viewed as exceptional promising sources of plant – based insecticides (Jacobson, 1989; Schmutterer, 1990). Entomo-toxic properties of extracts from plants belonging to several other families have also been frequently reported (Hermawan, et al., 1994; Sadek, 1997; Rodriguez – Saona and Trumble, 1999).
Moreover, it is well known that some insecticides from plants have been used for a long time; for instance, pyrethrum, obtained from the flower heads of C. cinerariifolium (=C. cinerariaefolium), was already known during the time of the Persian King Darius the Great (521-486 BC). Botanical insecticides are broad-spectrum in pest control, and many are safe to apply, unique in action, and can be easily processed and used. Botanical pesticides are biodegradable (Devlin and Zettel 1999). It also reduces environmental contamination and human hazards (Grainge and Ahmed, 1988).
Essential oils are believed to act as allelopathic agents or as irritants that protect plants from predation by insects and infestation by parasites (Simpson, 1995). Essential oils and their constituents have also been shown to be a potent source of botanical pesticides (Singh and Upadhyay, 1993). Jilani et al., (1988) reported that turmeric oil repelled various grain insects. Clove oil was shown to be toxic against Sitophilus oryzae (L.) and Rhyzopertha dominica (F.) (Sighamony et al., 1986). The essential oils of several spices like anise (Pimpinella anisum L.) and peppermint (Mentha piperita L.) have been found to have fumigant toxicity to four major stored product pests, R. domonica, Tribolium castaneum (Herbst), Oryzaephilus surinamensis (L.) and S. oryzae (Shaaya et al., 1991). Ho et al., (1996) found that the essential oil of garlic is insecticidal to T. castaneum and S. oryzae. The essential oils of nutmeg seed (Myristica fragrans Houtt) (Huang et al., 1997) and cinnamon bark (Cinnamomum aromaticum Nees) (Huang and Ho, 1998) are also toxic to T. castaneum and S. oryzae.
The Melaleuca essential oils have a high repellent effect upon the ant, Wasmannia auropunctata (Ochetomyrmex auropunctatus) which is a pest of both forest and plantations and fruit crops (Menendez et al., 1992). Alonso et al., (1996) demonstrated that undiluted cajuput oil inflicted 100% mortality to Andrector ruficornis (Cerotoma ruficornis) in lablab (Lablab purpureus cv. Rongai) in Cuba and has distinct repellent effects. Alonso – Anelot et al., (1994) found that essential oil of Melaleuca quinquenervia leaf has a potential for insect repellency on T. castaneum whereas the tea tree oil from Melaleuca alternifolia in water carrier could eliminate flea on dogs, cats, hamsters and white rats (Fitzjarrell, 1995).
Cajuput oil is also known to prevent plant infection of Herpes Simplex Type 1 on plant (Nawawi et al., 1999) and inhibits the growth of fungi, bacteria and yeast (De Colemnares et al., 1998; Dhirendra et al., 1989; Dubey et al., 1983; Farag et al., 1998).
Insect damage in stored grains may amount to 10% - 40% in countries where modern storage technologies have not been introduced (Shaaya et al., 1997). Therefore, there is a need for diverse kinds of safe insecticide or repellents for use on food grain. Generally, synthetic insecticides and fumigation are the main methods in the stored products pest control. However, increased public concern over the residual toxicity of insecticides applied to stored grain, the occurrence of insecticide resistant insect strains and the necessary precautions to work with traditional chemical insecticides calls for new approaches to control stored product insect pests. (Yildirim et al., 2001). Thus, there is a considerable interest in developing natural products that are relatively less damaging to the mammalian health and environment than existing conventional pesticides, as alternatives to non-selective synthetic pesticides to control the pests of medical and economic importance. (Tunc and Sahinkaya, 1998 and Keita et al., 2000).
M. leucadendron is easily found and grown in Southeast Asia countries. There is no report on the activities of M. leucadendron leaf essential oil against stored pests. So the purpose of this study is to investigate the activities of M. leucadendron against S. zeamais, one of the major stored pests in maize and T. castaneum, one of the major stored pests in groundnut.
Objectives
The objectives of this study are:
1. To study biological activity of Melaleuca essential oils on two stored pests, Sitophilus zeamais and Tribolium castaneum.
2. To increase the efficacy of Melaleuca essential oils using synergistic and/or formulation.
Literature Review
Sitophilus zeamais (Motschulsky)
Sitophilus zeamais (Maize weevil) is a small insect and belongs to order Coleoptera and family Curculionidae. It is a serious pest of stored grain throughout the warmer parts of the world. Infestation often starts in the field, and is later carried into the grain stored. The main host for this insect pest is maize and alternative hosts are sorghum, rice and other cereals.
Distribution
This pest is virtually cosmopolitan throughout the warmer parts of the world, extending as far north as Japan and southern Europe. It has been recorded from Europe (Spain, Italy, Turkey); Africa (Angola, Arabia, South Africa, East Africa, Ethiopia, Ghana, Madagascar, Madeira, Mauritius, Morocco, Mozambique, Nigeria); Asia (India, Tibet, Malaysia, Molucca Isles, Borneo, Japan); Australasia (Australia-New South Wales, Queensland, West Australia; New Guinea, New Zealand, Pacific Islands); USA (Texas, Florida); Central America (Costa Rica, Mexico, West Indies); South America (Argentina, Brazil, Guyana, Honduras, Chile, Equador, Guatemala, Nicaragua, Venezuela).
Life History
The female lays white and oval eggs inside the grain by chewing a minute hole in which each egg is deposited, followed by the sealing of the hole with a secretion. These eggs hatch into tiny grubs which stay and feed inside the grain and are responsible for most of the damage. Mature larvae are plump, legless and white, about 4 mm long. Pupation takes place inside the grain.
The adult beetle emerges by biting a circular hole through outer layers of the grain. They are small brown weevils, virtually indistinguishable from each other, about 3.5–4.0 mm long with rostrum and thorax large and conspicuous. The elytra are uniformly dark brown. Each female is capable of laying 300–400 eggs, and the adults live for up to 5 months and are capable fliers.
The life cycle is about 5 weeks at 30 ˚C and 70% RH; optimum conditions for development are 27–31˚C and more than 60% RH; below 17˚C development ceases (Dennis, 1983).
Damage
A thin tunnel is bored by the larva from the surface of the grain kernel. Circular exit holes on the surface of the grain kernel are characteristic.
Control
Infested buildings should be thoroughly cleaned and sprayed with BHC (Benzene Hexa Chloride) or malathion, and any parts of the building which cannot be reached with sprays should be fumigated with the use of recommended fumigants. Infested grtain can be mixed with malathion w.p. which is generally successful in achieving control.
Fumigation of infested grain with methyl bromide, or an ethylene dichloride and carbon tetrachloride mixture is effective but should only be carried out by approved operators because of the toxicity hazards.
Tribolium castaneum (Herbst)
Tribolium castaneum (red flour beetle) belongs to order Coleoptera and family Tenebrionidae. This species is a serious secondary pest throughout the warmer parts of the world in food stores. Infestation is apparently by the appearance of adults on the surface of the grain: there is extensive damage to previously holed or broken grains, or grain damage to previously damaged by other pests. Damage is done by both larvae and adults.
Distribution
This pest is cosmopolitan in warmer countries.
Life History
The female lays small, cylindrical and white eggs scattered in the produce. The larvae are yellowish-white, about 6 mm long when fully grown. The head is pale brown, and the last segment of the abdomen has two upturned dark pointed structures. The larvae live and develop inside the grain till pupation. The damage to the stored grains is done by the larvae. The pupa is yellowish-white, later becoming brown, the dorsum hairy, and the tip of the abdomen having two spine-like processes. The adult is rather flat, oblong, reddish-brown in colour, and about 3-4 mm long. Each female is capable of laying 400-500 eggs and the adults are long-lived, under some circumstances living for a year or more. The life period from egg to adult is 35 days at 30 ˚C. Adults fly in large numbers in the late afternoon (Dennis, 1983).
Damage
Infestation is apparent by the appearance of adults on the surface of the grain; there is extensive damage to previously holed or broken grains, or grain damaged by other pests. Damage is done by both larvae and adults.
Control
The shelled grain should be thoroughly admixed with BHC (Benzene Haxa Chloride) dust or powder, if locally permitted. Fumigation should only be carried out by approved operators.
Melaleuca leucadendron
The M. leucadendron is commonly known as cajuput tree, punk, weeping paperbark, milk wood, white wood, paper bark tree, swamp tea tree, tea tree, white tea tree and brown tea tree (Bailey, 1958). It is classified in the kingdom Plantae, class Magnoliopsida, order Myrtales and family Myrtaceae.
M. leucadendron is a large tree, native to Southeast Asia, the Pacific Islands and Australia. It can be found in many countries such as Indonesia, Malaysia, Vietnam, the Philippines, Thailand, Cuba, Venezuela, Turkey, United States and Hawaii (Alonso et al., 1996; De Colmenares et al., 1998; Kitanov et al., 1992). The tree can be used for many purposes such as essential oil, medicinal properties, fuel wood, timber, charcoal and shelter belts (Brinkman and Vo, 1991).
The oil of M. leucadendron leaf derived by steam distillation (known as cajuput oil or tea tree oil) is green in color with a camphor odor and is used medicinally such as antiseptic, stomachic, stimulant, analgesic, antirhematic, expectotant, and for treatment of intestinal worms (Usher, 1974; Kitanov et al., 1992). This essential oil contains 1, 8 – cineole, nerolidol, alloromadendrene, viridiflorol and α – terpineol as the major components (De Colmenares et al., 1998; Pino et al., 2002).
Botanical Insecticides
Several higher plants produce secondary metabolites that are considered as defensive phytochemicals inhibiting herbivory and infestation by microorganisms (Green and Hedin, 1986; Klocke, 1989; Rosenthal and Berenbaum, 1991). In addition, plant allelochemicals act as repellents, growth regulators and antifeedants (Jermy, 1990). Therefore, these plants may provide new sources of natural pesticides and antifeedants which can be used to replace heavy insecticides applications (Grainge and Ahmed, 1988; Ananthakrishnan, 1992). Antifeedant or feeding deterrent is often highly specific in action, and advantages in integrated pest management (IPM) applications (Whitehead and Bowers, 1983).
The indiscriminate use of chemical pesticides has given rise to many serious problems, including genetic resistance of pest species, toxic residues, increasing costs of application, environment pollution, hazards from handling etc. (Ahmed et al., 1981; Khanam et al., 1990). The development of cross- and multi-resistance strains in many important insect species, resulting from the continuous use of chemical insecticides, has been reported from all over the world (Dyte, 1970; Pasalu and Bhatia, 1983; Dyte and Halliday, 1985; Irshad and Gillani, 1990; Zettler and Cuperus, 1990; Zettler, 1991).
For several years, many plant species are being investigated for their natural products to be used as pest control. For example, some active ingredients of seeds and leaves of the tropical neem tree, Azadirachta indica, especially the tetranortriterpenoid azadirachtin, influence the feeding behavior, metamorphosis, fecundity and numerous insect species belonging to various orders (Jacobson, 1989). Neem oil, obtained by cold-pressing seed, can be effective against soft-bodied insects and mites but is also useful in the management of phytopathogens (Isman et al., 1996).
The use of neem leaves in protecting stored grain and other commodities is as age-old practice in India. Pruthi (1937) and Pruthi and Singh (1950) reported the efficacy of neem leaves in protecting stored grain from insect attack. Jotwani and Sircar (1965) showed that the neem kernel was effective as a grain protectant against stored grain insect pests.Yadav (1983) found that neem kernel protects leguminous seeds from attack by Callosobruchus maculatus (F.) and C. chinensis (L.)
Pandey et al., (1976) observed that 1 to 3 parts of neem oil per 100 parts of seed effectively protects Bengal gram (Cicer arietinum) seeds from damage by the bruchid C. chinensis for at least 135 days, without any adverse effects of treatment on germination. Ketkar (1976) reported the efficacy of neem kernel powder and neem oil in controlling the grain pests, under existing godong (warehouse) conditions.
Apart from those natural products in current use such as pyrethrins, rotenone, nicotine, ryania, sabadilla and neem oil, several substances of plant origin have been identified as having toxic, repellent, antifeedant, and/or growth- and development inhibiting-potential on arthropod pests (Coats, 1994). The potential of essential oils to act as fumigants has been demonstrated for stored-product pests (Klingauf et al., 1983; El-Nahal, et al., 1989; Singh et al., 1989;, Shaaya et al., 1991; Rice and Coats 1994; Saraç and Tunç 1995). These results indicate that essential oils may be efficacious and safe replacements for conventional synthetic fumigants.
In Togo, Adhikary (1981) compared neem leaf and seed powder with conventional insecticides for protecting corn stored in sacks or unpeeled corn cobs in bins against S. zeamais, Tribolium spp. R. dominica and Cathartus spp.
As spices are commonly used in cooking in various parts of the world, they are generally considered to be relatively harmless to humans compared with non-edible plants. In addition, spices possess various insect control properties. For example, acetone and hexane extracts of peppercorn are toxic to several species of stored product insects (Su, 1984). Chopped garlic and ethyl acetate extracts of garlic were found to repel T. castaneum and S. zeamais (Ho et al., 1987). The essential oil of garlic has also been demonstrated to be insecticidal to T. castaneum and S. zeamais (Ho et al., 1996). The essential oil of several spices, such as anise (P. anisum) and peppermint (M. piperita) have fumigant toxicity to four major stored product pests, R. dominica, T. castaneum, S. oryzae and O. surinamensis (Ho et al., 1994) and star anise (Illicium verum Hook F) (Ho et al., 1995) showed insecticidal to T. castaneum and S. zeamais and suppressed progeny production. The main constituent of star anise, anethole, was also insecticidal to these two stored product insects and suppressed progeny production (Ho et al., 1997).
The addition of acetone extracts of black pepper and nutmeg seeds reduced the number of F1 progeny and prolonged the developmental period of S. zeamais (Haryadi and Rahayu, 2003). Himalayan cedarwood oil drilled from the wood chips of Cedrus deodara was found to possess its toxicity to the pulse beetle (Singh and Rao, 1985) and protection against rice weevil (Singh et al., 1989).
Materials and Methods
Insects
Two species of insects from Department of Agriculture, Ministry of Agriculture and Co-operative, Thailand will be used in this study: S. zeamais (maize weevil) and T. castaneum ( red flour beetles). S. zeamais will be reared on rice 12–13% moisture content while T. castaneum will be reared on rice bran. The cultures will be maintained in the laboratory at 29-32 ˚C and 70–80% RH.
Extraction of Essential Oils
Fresh leaves or fruits of selected plants will be collected from several places or bought from local market, where no pesticides are applied. The fresh leaves will be cut into small pieces and placed in 5 l extracting flask before steaming on the heating mantle for 6–8 h. Then, essential oil will be collected and kept in the refrigerator for further use.
Repellency Test
Petri dishes of 9 cm in diameter will be used to confine insects during experiment. Melaleuca essential oil will be diluted in ethanol to different concentrations (1%, 2%, 3% and 4%) and ethanol absolute will be used as control. Filter papers (9 cm diameter) will be cut in half. One ml of Melaleuca essential oil will be applied to one half of the filter paper as uniformly as possible with a micropipette. The other half (control) will be treated with 1 ml of ethanol absolute. Both treated half and control sides will then be air–dried to evaporate the solvent completely. Full disc will be carefully remade by attaching tested halves to control halves with sellotape. Precautions should be taken so that attachment will not prevent the free movement of insects from one half to another, but the distance between the filter-paper halves remained sufficient to prevent seepage of test samples from one half to another. Each remade filter paper will be placed in a petri dish with the seam oriented in one of four randomly selected different directions to avoid any insecticidal stimuli affecting the distribution of insects. Ten insects will be released at the center of each filter-paper disc and a cover will be placed on the petri dish. For each essential oil, five replicates will be used and the experiment will be repeated once. Counts of the insects present on each strip will be made after 1 h and at hourly interval up to the fifth hour.
Fumigant Toxicity
Filter papers (2 cm diameter) will be impregnated with aliquots of 25 µl of an appropriate concentration of Melaleuca essential oil. Ethanol absolute will be used as control. After evaporating the solvent or ethanol for 2 min in air, each filter paper will be placed on the underside of the screw cap of a glass vial (2.5 cm diameter, 5.5 cm height). The cap will be screwed tightly onto the vial containing ten adults of either species of insects. Each concentration and control will be replicated five times, and the experiment will be repeated once. After 24 h, the insects will be transferred to holding cages with culture medium in incubator for the determination of end-point LC50 and LC95 values.
Contact Toxicity
The filer paper impregnation method will be used to examine the contact toxicity. Filter papers (9 cm diameter) will be impregnated with aliquots of 0.5 ml of an appropriate concentration of different essential oils. Ethanol absolute will be used for the control in these experiments. The filter papers will be air dried for 1 h. A glass ring (5.4 cm diameter, 2.5 cm depth) will be placed on each filter paper before 20 insects will be confined to each glass ring. Sticky substance will be applied to the inner surface of each ring to prevent the insects from climbing onto the side of the ring. For adult S. zeamais and T. castaneum, five replicates will be set up for each essential oil concentration and control, and experiment will be repeated once and kept them till new generation.
Flour Disk Bioassay
Aliquots of 200 µl of a suspension of wheat flour in water (10 g in 50 ml) will be dropped onto a clean plastic placed in a tray. The disks will be left in the fume hood overnight to dry, after which they will be put in an oven at 60 ˚C for 24 h. Melaleuca essential oil will be diluted in ethanol to get different concentrations (2%, 4% and 6%) and ethanol absolute and no ethanol will be used as two controls. After evaporation of the solvent, the disks will be placed in glass vials (diameter 2.5 cm, height 5.5 cm). Ten group-weighed, unsexed adults will be added to each preweighed vial containing the disk. Five replicates will be prepared. After three days, the glass vials with flour disks and live insects will be weighed again, and mortality of insects, if any, will be recorded. In order to eliminate the decrease in weight of flour disks due to the evaporation of ethanol and Melaleuca essential oil, the weight of one and two flour disks treated with ethanol or various concentrations of Melaleuca essential oil will be recorded. After three days, the disks will be weighed again to determine the decrease in their weight. The weight of the flour disks used in the experiments will be corrected for any decrease in weight due to evaporation and drying. Nutritional indices will be calculated with some modifications: relative growth rate (RGR) = (A-B)/B x day-1, where A = weight of live insects on the third day (mg)/No. of live insects on the third day, B = original weight of insects (mg)/No. of insects; relative consumption rate (RCR) = D/B x day-1, where D = biomass ingested (mg)/No. of live insects on the third day; efficiency of conversion of ingested food (ECI)(%) = (RGR/RCR) x 100. For antifeedant action, the formula described by Isman et al. (1990) will be modified in calculating the feeding deterrence index (FDI)(%) = (C-T)/C x 100, where C = the consumption of control disks and T = the consumption of treated disks, as the control and treated disks will be placed in separate vials in no-choice tests.
Data Analysis
Data in all experiment will be analyzed by SAS program, and Least Significant Difference Test (LSD) will be analyzed to compare the treatment means (P <>Experimental Place and Duration
Department of Entomology, Faculty of Agriculture, Kasetsart University, Bangkok, Thailand (June - 2007 to Jan - 2009).
Study Supported
All of these studies are under financial support by the government of The Union of Myanmar under technical support by FAO for Myanmar Oil Crops Development Project.
Literature Cited
Abbot, W. S. 1925. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 18: 265-267.
Adhikary, S. 1981. Togo experience in moving from neem research to its practical application for plant protection. pp. 215-222. In H. Schmutterer., K.P.R. Ascher and H. Rembold, eds. Proc. 1st Int. Neem conf. (Rotach-Egern, Germany, 1980).
Ahmed, S. M., H. Chander and J. Pereira. 1981. Insecticidal potential and biological activity of Indian indigenous plants against Musca domestica. Int. Pest Control. 23: 170-175.
Aliaga, T. J and Feldheim, W. 1985. Hemmung der Keimbildung bei gegagerten Kartoffeln durch das ätherische older südamerikanischen Munapflanze (Minthostachys spp.) Ernährung 9: 254-256.
Alonso, O. S., M. D. C. Sanchez and A. Delgado. 1996. The oil extract Cajeput, a repellent and bio-insecticide against Andrector ruficornis. Pastosy Forrajes 19: 289-293.
Alonso-Anelot, M. E., J. L. Avila, L. D. Otero, F. Mora and B. Wolff. 1994. A new bioassay for testing plant extracts and pure compounds using red flour beetle Tribolium castaneum Herbst. J. Chem. Ecol. 20: 1161-1177.
Ananthakrisnan, T. N. 1992. Dimensions of Insect-Plant Interactions, Oxford and IBH Publishing, New Delhi.
Bailey, L. H. 1958. The standard cyclopedia of horticulture Macmillan press, New York.
Bell, E. A., L. E. Fellows and M. S. J. Simmonds. 1990. Natural products from plants for the control of insect pests. pp. 337-350. In E. Hodgson and R. J. Kuhr (eds.). Safer Insecticides. M. Dekkar, New York.
Breuer, M. and G. H. Schmidt. 1995. Influence of a short period treatment with Melia azedarach extract on food intake and growth of the larvae of Spodoptera frugiperdu (Smith) (Lepidoptera: Noctuidae). J. Pl. Dis. Prot. 102: 633-654.
Brinkman, W. J. and T. X. Vo. 1991. Melaleuca leucadendron, a useful and versatile tree for acid sulphate soils and some other poor environments. J. Int. Tree Crops 6: 261-274.
Coats, J. R. 1994. Risks from natural versus synthetis insecticides. Annu. Rev. Entomol. 39: 489-515.
De Colmenares, N. G., G. O. De Rodriguez, A. Prieto, O. Crescente and L. Cabrera. 1998. Phytoconstituents and antimicrobial activity of Melaleuca leucadendron leaf essential oil from Venezuela. Ciencia (Maracabo) 6: 123-128.
Dennis, S. H. 1983. Agricultural Insect Pests of the Tropics and Their Control (2nd eds.). Cambridge University Press, Cambridge, New York, 437 p.
Devlin, J. F and T. Zettle. 1999. Ecoagriculture: Initiative in Eastern and Sothern Africa. Weaver Press. Harare.
Dhirendra, M., N. Misra and D. Misra. 1989. Antifungal activity of cajeput oil against Aspergillus fumigatus (EIDAM) Wint. (NRRL 1979) and Fusarium moniliforme Sheldon (NRRL 6398). Indian Perf. 33; 151-155.
Dimock, M. B and J. A. A. Renwick. 1991. Oviposition by field populations of Pieris rapae (Lepidoptera: Pieridae) deterred by an extractant of a wild crucifer. Environ. Entomol. 20: 802-806.
Dubey, N. K., N Kishore, S. K. Singh and A. Dikshit. 1983. Antifungal properties of the volatile fraction of Melaleuca leucadendron. Trop. Agric. 60: 227-228.
Dyte, C. E. 1970. Insecticide resistance in stored-product insects with special reference to Tribolium castaneum. Trop. Stored Prod. Inf. 20: 13-15.
Dyte, C. E. and D. Halliday. 1985. Problems of development of resistance to phosphine by insect pests of stored grains. Bull. Org. Europe. Mediter. Prot. Plant. 15: 51-57.
El-Ibrashy, M. T. 1974. Sterilization of the Egyptian cotton leafworm Spodoptera littoralis (Boisd.) with a foliage extract of Podocarpus gracilior J. Appl. Entomol. 75: 107-109.
El-Nahal, A. K. M., G. H. Schimidt, and E. M. Risha. 1989. Vapours of Acorus calamus oil-a space treatment for stored-product insects. J. Stor. Prod. Res. 25: 211-216.
Farag, R. S., Z. Y. Daw, M.A.S. Mahassen and H. M. Saffaa. 1998. Biochemical and biological on some tea trees (Melaleuca spp.) essential oils. Adv. Food Sci. 20: 153-162.
Finney, D. J. 1971. Probit Analysis, University press, Cambridge, UK. 333 p.
Fitzjarrell, E. A. 1995. Method and formulation for elimination fleas on animals-USPTO Patent full-text and image database. United States Patent. 5,449,517, United States.
Grainge, N. and S. Ahmed. 1988. Handbook of Plants with Pest Control Properties. Wiley. New York.
Green, M. B and P. A. Hedin. 1986. Natural Rresistance of Plants to Pests: Role of Allelochemicals. ACS symposium Series. American Chemical Society, Washinton DC.
Haryadi, Y. and S. Rahayu. 2003. Study on the effects of mixture of acetone extracts of black pepper (Piper nigrum L.) and Nutmeg (Myristica fragrans Houtt) seeds on the development of Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). pp. 863-865. In P.F. Credland, D. M. Armitage, C.H. Bell. P. M. Cogan and E. Highley eds. Advances in Stored Product Protection, Proc. of the 8th Int. Working Conference on Stored Product Protection (2003). 22-26 July 2002. York, UK.
Hermawan, W., S. Kojiyama, R. Tsukuda, K. Fujisaki, A. Koboyashi and F. Nakasuji. 1994. Antifeedant and antioviposition activities of fraction of extract from a tropical plant Andrographis paniculata (Acanthaceae) against the diamondback moth, Plutella xylostella (Lepidoptera: Yponomeutidae). Appl. Entomol. Zool. 29: 533-538.
Herve, B. D. 1981. La munia como insecticida casero. Contribusion Regional Tecnica Apropriada, Bolivia, pp. 24-25.
Hiremath, I. G., Y. J. Ahn, and S.I. Kim. 1997. Insecticidal activity of Indian plant extracts against Nilaparvata lugens (Homoptera: Delphacidae). Appl. Entomol. Zool. 32: 159-166.
Ho, S. H., Y. Ma and H. T. W. Tan. 1987. Repellency of some plant extracts to the stored products beetles, Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch. Proc. Symp. Pest Management Stored Food and Feed, SEMEO BIOTROP, Bogor, Indonesia, September, 1995.
Ho, S. H., L. P. L. Cheng, K. Y. Sim and H. T. W. Tan. 1994. Potential of clove (Syzygium aromaticum (L.) Merr. and Perry as a grain protectant against Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch. Postharvest Biol. Tech. 4: 179-183.
Ho, S. H., Y. Ma and K. Y. Sim. 1995. Star anise, Illicium verum Hook f., as a potential grain protectant against Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch. Postharvest Biol Tech. 6: 341-347.
Ho, S. H., L. Koh, Y. Ma, Y. Huang and K. Y. Sim. 1996. The oil of garlic, Allium sativum L. (Amaryllidaceae), as a potential grain protectant against Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch. J. Stor. Prod. Res. 34: 11-17.
Ho, S.H., Y. Ma and Y. Huang. 1997. Anethole, a potential insecticide from Illicium verum Hook f., against two stored product insects. Int. Pest Control. 39: 50-51.
Huang, Y and S. H. Ho. 1998. Toxicity and antifeedant activities of cinnamaldehyde against the grain storage insects, Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch. J. Stored Prod. Res. 34: 11-17.
Huang, Y., J. M. W. L. Tan, R. M. Kini and S. H. Ho. 1997. toxic and antifeedant action of nutmeg oil against Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch. J. Stored Prod. Res. 33: 289-298.
Irshad, M. 1989. Botanical insecticides Past, present and future. pp. 1-10. In Insecticides of Plant Origin. J. T. Arnason., B. J. R. Philogène and P. Morand (eds.). American Chemical Society Symposium Series No. 387, Washinton DC. American Chemical Society.
Irshad, M. and W. A. Gillani. 1990. Resistance in Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) against malathion, Pakistan J. Zool. 22: 257-262.
Isman, M. B., O. Koul, A. Luczynski and J. Kaminski. 1990. Insecticidal and antifeedant bioactivities of neem oils and their relationship to azadirachtin content. J. Agric. Food Chem. 38: 1406-1411.
Isman, M. B., H. Matsuura, S. MacKinnon, T. Durst, G. H. N. Towers and J. T. Arnason. 1996. Phytochemistry of the Meliaceae. So many terpenoids, so few insecticides. pp. 155-178. In Phytochemical Diversity and Redundacy. J. T. Romeo., J. A. Saunders and P. Barbosa (eds.) New York: Plenum.
Jacobson, M. 1989. Botanical pesticides: past, present and future. In Insecticide of Plant Origin. American Chemical Society, Washington DC.
Jermy, T. 1990. Prospects of antifeedant approach to pest control – a critical review. J. Chem. Ecol. 16: 3151-3166.
Jilani. G., R. C. Sazena and B. P. Rueda. 1988. Repellent and growth inhibiting effects of turmeric oil, sweetflag oil, neem oil and “Margosan-O” on red flour beetle (Coleoptera: Tenebrionidae). J. Econ. Entomol. 81: 1226-1230.
Jotwani, M. G. and P. Sircar. 1965. Neem seed as a protectant against stored grain pests infesting wheat seed. Indian J. Entomol. 27: 161-164.
Keita, S. M., C. Vincent, J. P. Schmit, S. Ramaswamy and A. Belanger. 2000. Effect of various essential oils on Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). J. Stor. Prod. Res. 36: 355-364.
Ketkar, C. M. 1976. Final Technical Report ‘Utilization of Neem (Azadirachta indica A. Juss) and its Byproducts’. Directorate of Non-edible Oils and Soap Industry, Khada and Village Industries Commision, Bombay, pp. 150-152.
Khan, Z. R. and R. C. Saxena. 1986. Effect of steam distillate extracts of resistant and susceptible rice cultivars in behavior of Sogatella furcifera (Homoptera: Delphacidae). J. Econ. Entomol. 79: 928-935.
Khanam, L. A. M., D. Talukder, A. R. Khan and S. M. Rahman. 1990. Insecticidal properties of Royna, Aphanamixis polysachya Wall. (Paker) (Meliaceae) against Tribolium confusum Duval. J. Asiat. Soc. Bangladesh Sci. 16: 71-74.
Kitanov, G. M., T. V. Dam, I. Assenov and T. Dam. 1992. Flavonols from Melaleuca leucadendron leaves, Fitoterapia. 63: 379-380.
Klepzig, K. D and F. Schlyter. 1999. Laboratory evaluation of plant derives antifeedants against the pine weevil Hylobious abictis (Coleoptera: Curculionidae). J. Econ. Entomol. 92: 644-650.
Klingauf, F., H. J. Bestman, O. Vostrovsky and K. Michaelis. 1983. Wirkung von ätherischen Ölen auf Schadinsekten. Mitteilungen der Deuschen Gesellschaft für Allgemeine und Angewandte Entomologie. 4: 123-126.
Klocke, J. 1989. Economic and medicinal plant research. In H. Wagner, J. Hikino and N. R. Farnsworth, eds. Plant compounds as models for insect control agents. Academic Press, New York.
Madrid, F.J., N.D.G. White and S.R. Loschiavo. 1990. Insects in stored cereals and their association with farming practices in Southern Manitoba. Can. Entomolo. 122: 515–523.
Menendez, J. M., M. D. C. Berrios and R. Quert. 1992. Preliminary study on the repellent effect of the essential oils of three species of the Myrtaceae on Wasmannia auropunctata. Revista Baracoa. 22: 47-50.
Metcalf, C.L., W. P. Flint and R. L. Metcalf. 1992. Destructive and useful insects. Mcgraw-Hill, New York.
Muthukrishnan, J. and E. Pushpalatha. 2001. Effects of plant extracts on fecundity and fertility of mosquitoes. J. Appl. Entomol. 125: 31-35.
Nawawi, A., N. Nakamura, M. Hattori, M. Kurokawa and K. Shiraki. 1999. Inhibitory effects of Indonesian medicinal plants on the infection of Herpes Simplex Virus Type I. Phytother. 13: 37-41.
Pandey, N. D., R. S. Shiva and G. C. Tiwari. 1976. Use of some plant powders, oils and extracts as protectant against the pulse beetle, Callosobruchus chinensis L. Indian J. Entomol. 38: 110-113.
Papachristos, D. P and D. C. Stamopoulos. 2002. Toxicity of vapors of three essential oils to the immature stages of Acanthoscelides obtectus (Say) (coleopteran: Bruchidae). J. Stored Prod. Pes. 38: 365-373.
Pasalu, I. C and S. K. Bhatia. 1983. Inheritance of resistance to malathion in Tribolium castaneum (Herbst). Proc. Indian Acad. Sci. (Anim. Sci.). 92: 409-414.
Perkins, J. H. 1985. Naturally occurring pesticides and the pesticide crisis. 1945 to 1980. pp. 297-325. In N. B. Mandava, ed. Handbook of Natural Pesticides: Methods. Vol. 1: Theory, Practice and Detection. CRC. Boca. FL.
Pino, J., A. Bello, J. Urquiola, J. Aguero and R. Marbot. 2002. Chemical composition of cajuput oil (Melaleuca leucadendron L.) from Cuba. J. Esocent. Oil Res. 14: 10-11.
Pruthi, N. S. 1937. Report of the Imperial Entomologist, Science. Rept. Agric. 1st. New Delhi, India. 1935-36.
Pruthi, N. S and M. Singh. 1950. Pests of stored grain and their control. Indian J. Agric. Sci. 18 (4): 52 (special No. Vol. 18, 1948).
Rembold, H. 1988. Isomeric azadirachtins and their mode of action. pp. 47-67. In M. Jacobson, ed. Phytochemical Pesticides. Vol.1. The Neem Tree. CRC, Boca Raton.
Rice, P. J and J. R. Coats. 1994. Insecticidal properties of several monoterpenoids to the housefly (Diptera: Muscidae), red flour beetle (Coleoptera: Tenebrionidae) and southern corn rootworm (Coleoptera: Chrysomelidae). J. Econ. Entomol. 87: 1172-1179.
Rodriguez-Saona, C. R. and J. T. Trumble. 1999. Effect of ovocardofurans on larval survival, growth and food preference of the generalist herbivore, Spodoptera exigua. Entomol. Expt. Appl. 90: 131-140.
Rosenthal, G. A. and M. R. Berenbaum. 1950. Pests of stored grain and their control. Indian J. Agric. Sci. 18 (4): 52 (special Number Vol: 18, 1948)
Sadek, M. M. 1997. Antifeedant and larvicidal effects of Eichornia crassipes leaves on the cotton leafworm Spodoptera littoralis (Boisd.). J. Egypt.Ger. Soc. Zool. 24 (E): 209-232.
Saraç, D. and I. Tunç. 1995. Toxicity and repellency of essential oils to stored-product insects. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz. 102: 429-434.
Schmutterer, H. 1990. Properties and potential of natural pesticides from the neem tree. Ann. Rev. Entomol. 35: 271-297.
Shaaya, E., M. Kostjukovski, J. Eilberg and C. Sukprakarn. 1997. Plant oils as fumigants and contact insecticides for the control of stored-product insects, J. Stor. Prod. Res. 33: 7-15.
Shaaya, E., U. Ravid, N. Paster, B. Juven and U. Zisman. 1991. Fumigant toxicity of essential oils against four major stored-product insects. J. Chem. Ecol. 17: 499-504.
Sharaby, A. 1988. Evaluation of some Myrtaceae plant leaves as protectant against the infestation by Sitophilus oryzae L. and Sitophilus granarius L. Insect Sci. Appl. 9: 465-468.
Singh, B. B and R. K. Upadhyay. 1993. Essential oils: a potent source of natural pesticides. J. Sci. Indus. Rs. 52: 676-683.
Sighamony, S., I. Anees, T. Chandrakala and J. Osmani. 1986. Efficiency of certain indigenous plant products as grain protectants against Sitophilus oryzae (L.) and Rhyzopertha dominica (F.) J. Stored Prod. Res. 22: 21-23.
Simpson. B. B. 1995. Spices, herbs and perfumes. pp. 278-301. In B. B. Simpson and M. C. Ogorzaly, eds. York.
Singh, D. and S. M. Rao. 1985. Toxicity of cedarwood oil against pulse beetle, Callosobruchus chinensis Linn. Indian Perf. 29: 201-204.
Singh, D., M. S. Siddiqui and S. sharma. 1989. Reproduction retardant and fumigant properties in essential oils against rice weevil (Coleoptera: Curculionidae) in stored wheat. J. Econ. Entomol. 82: 727-733.
Spurgeom, D. 1977. Hidden Harvest: A system approach to post-harvest technology. PAG Bull. 7: 45-48.
Su, H. C. F. 1984.Comparative toxicity of three peppercorn extracts to four species of stored-product insects under laboratory conditions. J. Georgia Entomol. Soc. 19: 190-199.
Tripathi, A.K. V. Prajapati, K. K. Aggarwal and S. Kumar. 2001. Toxicity, Feeding deterrence and effect of activity of 1,8 cineole from Artemisia annua on progeny production of Tribolium castaneum (Coleoptera: Tenebrionidae). J. Econ. Entomol. 94: 979–983.
Tunc, I. and S. Sahinkaya. 1998. Sensitivity of two greenhouse pests of vapours of essential oils. Entomol. Exp. Appl. 86: 183-187.
Usher, B. 1974. Dictionary of Plants Used by Man. Constable and Co., London.
Ware, G. W. 1986. Fundamentals of pesticides. Thompson Publishing Frenco. Ca.
Warthen, J. D., Jr. 1979. Azadirachta indica: a source of insect feeding inhibitors and growth regulators. U.S. Dept. Agric. Rev. Mon. ARM-NE-4.
Weiss, E. A. 1997. Litsea cubeta. In Essential oil crops. CAB International, Wallingford, Oxon Ox 10 820, UK. pp. 207.
Wheeler, D. A. and M.B. Isman. 2000. Effect of Trichilia American extract on feeding behavior of Asian armyworm, Spodoptera litura, J. Chem. Ecol. 26: 2791-2800.
Whitehead, D. L. and W. S. Bowers. 1983. Natural products for innovative pest management. Pergamon. Oxford.
Yadav, T. D. 1983. Studies on the Insecticidal Treatment Against Bruchids Callosobruchus maculatus (Fabr.) and C. chinensis L. Damaging Stored Leguminous Seeds Ph. D. thesis, Ara University, Agra (U.P), India.
Yidirim, E., H. Ozbek and I. Aslan. 2001. Pests of stored product, Ataturk University Agricultural Faculty Press (2001). No.191. pp. 117
Zettler, J. L. and G. W. Cuperus. 1990. Pesticide resistance in Tribolium castaneum (Coleoptera: Tenebrionidae) and Rhizopertha domineca (Coleoptera: Bostrichidae) in wheat. J. Entomol. 83: 1677-1681.
1995.
Zettler, J. L. 1991. Pesticide resistance in Tribolium confusum (Coleoptera: Tenebrionidae) from flour mills the USA. J. Econ. Entomol. 84: 763-767.
Zhao, B., G. G Grant, D. Langevin, and L. Macdonald. 1998. Deterring and inhibiting effects of quinizidine alkaloids in spruce budworm (Lepidoptera: Tortricidae) oviposition. Environ. Entomol. 27: 984-992.
Introduction
Insect pests cause heavy losses of stored grain quantitatively and qualitatively throughout the world (Madrid et al., 1990). Insect damage in stored grains may amount to 10% - 40% in countries where modern storage technologies have not been introduced (Shaaya et al., 1997). Insect pests cause the loss of stored food grain in tropical and semitropical environments (Tripathi et al., 2001). Chemical control of insects in storage has been used for a long time, but has serious drawbacks (Sharaby, 1988). Careless and indiscriminate use of synthetic pesticides has led to a number of wellknown problems. Some of these are contamination of food, soil, ground water, rivers, lakes, oceans, air etc. with toxic residues, side effects on non-target insects and other organisms, increase of the number of pest species resistant to pesticides, and pest resurgence. In addition, many non-lethal as well as lethal accidents occurred due to mishandling of highly toxic synthetic products.
As a logical consequence of the undesirable side effects of these products, there is a growing awareness in industrialized and also in developing countries, of the toxicological and environmental problems involved in the use of synthetic pesticides. This awareness has led to a steadily increasing movement towards a more environmental – oriented, sustainable agriculture with low or no input of toxic synthetic pesticides and other agricultural chemicals in an attempt to preserve and protect the environment as well as human health.
The plant kingdom is by far the most efficient “factory” of compounds. It synthesizes secondary metabolic products which can partly be considered as weapons to defend plants against pests and diseases which have competed with them since time immemorial.
Historically, chemicals derived from plants have been an important source of insecticides. The insecticidal properties of the alkaloid nicotine, for example, have been known since 1800s (Metcalf et al., 1962; Ware, 1986). Botanical materials such as pyrethrum, rotenone, and nicotine were among the first compounds used to control agricultural insect pests (Perkins, 1985; Grainge and Ahmed, 1988). With the advent of effective and economical synthetic insecticides, however, plant-based insecticides used in agriculture were displaced. As technological (resistance, resurgence and secondary outbreaks) and cultural (health and environmental) problems involved in the use of synthetic pesticides have increased, interest in naturally occurring plant compounds as alternative sources of pest control has been renewed (Perkins, 1985). Currently, novel, as well as established, secondary plant compounds is being evaluated as alternatives for crop protection in many countries.
Several commercial produced plant-derived compounds have exhibited good potential as insecticides. Azadirachtin, a triterpenoid from the neem tree, Azadirachta indica A. Juss (Meliaceae), has growth-regulating and antifeedent properties against numerous insect pests (Warthen, 1979; Rembold, 1988). Commercial preparations of many household insecticides are augmented with pyrethrins, a complex of esters extracted from flowers of Chrysanthemum cinerariefolium (Treviranus) (Compositae) (Bell et al., 1990). Alkaloids that have been used commercially include ryanodine, the active ingredient in ryania (Ryania spp., Flacourtiaeae) the principal components extracted from the seeds of sabadilla, Schoenocaulon spp., Liliaceae (Ware 1986; Bell et al., 1990). Although too expensive for commercial synthesis, rotenone, an isoflavonoid procured primarily from two genera of tropical legumes, several Derris and Lonchocarpus spp., (Fabaceae), has been moderately effective against insects with chewing mouthparts (Bell et al., 1990).
Plants are rich sources of natural substances that can be utilized in the development of environmentally safe methods for insect control. The deleterious effects of certain purified phytochemicals or crude plant extracts on insects are manifested in several ways including toxicity (Hiremath et al., 1997), growth retardation (Breuer and Schmidt, 1995), feeding inhibition (Klepzig and Schlyter, 1999; Wheeler and Isman, 2000), oviposition deterrence (Dimock and Renwick, 1991; Hermawan et al., 1994; Zhao et al., 1998), suppression of calling behavior (Khan and Saxena, 1986) and reduction of fecundity and fertility (El-Ibrashy,1974; Muthukrishnan and Pushpalatha, 2001). Although a wide variety of effects provide potential alternatives for the use of synthetic chemical insecticides, certain plant families, particularly Meliaceae, Asteraceae, Rutaceae, Labiaceae, Annonaceae and Canellaceae are viewed as exceptional promising sources of plant – based insecticides (Jacobson, 1989; Schmutterer, 1990). Entomo-toxic properties of extracts from plants belonging to several other families have also been frequently reported (Hermawan, et al., 1994; Sadek, 1997; Rodriguez – Saona and Trumble, 1999).
Moreover, it is well known that some insecticides from plants have been used for a long time; for instance, pyrethrum, obtained from the flower heads of C. cinerariifolium (=C. cinerariaefolium), was already known during the time of the Persian King Darius the Great (521-486 BC). Botanical insecticides are broad-spectrum in pest control, and many are safe to apply, unique in action, and can be easily processed and used. Botanical pesticides are biodegradable (Devlin and Zettel 1999). It also reduces environmental contamination and human hazards (Grainge and Ahmed, 1988).
Essential oils are believed to act as allelopathic agents or as irritants that protect plants from predation by insects and infestation by parasites (Simpson, 1995). Essential oils and their constituents have also been shown to be a potent source of botanical pesticides (Singh and Upadhyay, 1993). Jilani et al., (1988) reported that turmeric oil repelled various grain insects. Clove oil was shown to be toxic against Sitophilus oryzae (L.) and Rhyzopertha dominica (F.) (Sighamony et al., 1986). The essential oils of several spices like anise (Pimpinella anisum L.) and peppermint (Mentha piperita L.) have been found to have fumigant toxicity to four major stored product pests, R. domonica, Tribolium castaneum (Herbst), Oryzaephilus surinamensis (L.) and S. oryzae (Shaaya et al., 1991). Ho et al., (1996) found that the essential oil of garlic is insecticidal to T. castaneum and S. oryzae. The essential oils of nutmeg seed (Myristica fragrans Houtt) (Huang et al., 1997) and cinnamon bark (Cinnamomum aromaticum Nees) (Huang and Ho, 1998) are also toxic to T. castaneum and S. oryzae.
The Melaleuca essential oils have a high repellent effect upon the ant, Wasmannia auropunctata (Ochetomyrmex auropunctatus) which is a pest of both forest and plantations and fruit crops (Menendez et al., 1992). Alonso et al., (1996) demonstrated that undiluted cajuput oil inflicted 100% mortality to Andrector ruficornis (Cerotoma ruficornis) in lablab (Lablab purpureus cv. Rongai) in Cuba and has distinct repellent effects. Alonso – Anelot et al., (1994) found that essential oil of Melaleuca quinquenervia leaf has a potential for insect repellency on T. castaneum whereas the tea tree oil from Melaleuca alternifolia in water carrier could eliminate flea on dogs, cats, hamsters and white rats (Fitzjarrell, 1995).
Cajuput oil is also known to prevent plant infection of Herpes Simplex Type 1 on plant (Nawawi et al., 1999) and inhibits the growth of fungi, bacteria and yeast (De Colemnares et al., 1998; Dhirendra et al., 1989; Dubey et al., 1983; Farag et al., 1998).
Insect damage in stored grains may amount to 10% - 40% in countries where modern storage technologies have not been introduced (Shaaya et al., 1997). Therefore, there is a need for diverse kinds of safe insecticide or repellents for use on food grain. Generally, synthetic insecticides and fumigation are the main methods in the stored products pest control. However, increased public concern over the residual toxicity of insecticides applied to stored grain, the occurrence of insecticide resistant insect strains and the necessary precautions to work with traditional chemical insecticides calls for new approaches to control stored product insect pests. (Yildirim et al., 2001). Thus, there is a considerable interest in developing natural products that are relatively less damaging to the mammalian health and environment than existing conventional pesticides, as alternatives to non-selective synthetic pesticides to control the pests of medical and economic importance. (Tunc and Sahinkaya, 1998 and Keita et al., 2000).
M. leucadendron is easily found and grown in Southeast Asia countries. There is no report on the activities of M. leucadendron leaf essential oil against stored pests. So the purpose of this study is to investigate the activities of M. leucadendron against S. zeamais, one of the major stored pests in maize and T. castaneum, one of the major stored pests in groundnut.
Objectives
The objectives of this study are:
1. To study biological activity of Melaleuca essential oils on two stored pests, Sitophilus zeamais and Tribolium castaneum.
2. To increase the efficacy of Melaleuca essential oils using synergistic and/or formulation.
Literature Review
Sitophilus zeamais (Motschulsky)
Sitophilus zeamais (Maize weevil) is a small insect and belongs to order Coleoptera and family Curculionidae. It is a serious pest of stored grain throughout the warmer parts of the world. Infestation often starts in the field, and is later carried into the grain stored. The main host for this insect pest is maize and alternative hosts are sorghum, rice and other cereals.
Distribution
This pest is virtually cosmopolitan throughout the warmer parts of the world, extending as far north as Japan and southern Europe. It has been recorded from Europe (Spain, Italy, Turkey); Africa (Angola, Arabia, South Africa, East Africa, Ethiopia, Ghana, Madagascar, Madeira, Mauritius, Morocco, Mozambique, Nigeria); Asia (India, Tibet, Malaysia, Molucca Isles, Borneo, Japan); Australasia (Australia-New South Wales, Queensland, West Australia; New Guinea, New Zealand, Pacific Islands); USA (Texas, Florida); Central America (Costa Rica, Mexico, West Indies); South America (Argentina, Brazil, Guyana, Honduras, Chile, Equador, Guatemala, Nicaragua, Venezuela).
Life History
The female lays white and oval eggs inside the grain by chewing a minute hole in which each egg is deposited, followed by the sealing of the hole with a secretion. These eggs hatch into tiny grubs which stay and feed inside the grain and are responsible for most of the damage. Mature larvae are plump, legless and white, about 4 mm long. Pupation takes place inside the grain.
The adult beetle emerges by biting a circular hole through outer layers of the grain. They are small brown weevils, virtually indistinguishable from each other, about 3.5–4.0 mm long with rostrum and thorax large and conspicuous. The elytra are uniformly dark brown. Each female is capable of laying 300–400 eggs, and the adults live for up to 5 months and are capable fliers.
The life cycle is about 5 weeks at 30 ˚C and 70% RH; optimum conditions for development are 27–31˚C and more than 60% RH; below 17˚C development ceases (Dennis, 1983).
Damage
A thin tunnel is bored by the larva from the surface of the grain kernel. Circular exit holes on the surface of the grain kernel are characteristic.
Control
Infested buildings should be thoroughly cleaned and sprayed with BHC (Benzene Hexa Chloride) or malathion, and any parts of the building which cannot be reached with sprays should be fumigated with the use of recommended fumigants. Infested grtain can be mixed with malathion w.p. which is generally successful in achieving control.
Fumigation of infested grain with methyl bromide, or an ethylene dichloride and carbon tetrachloride mixture is effective but should only be carried out by approved operators because of the toxicity hazards.
Tribolium castaneum (Herbst)
Tribolium castaneum (red flour beetle) belongs to order Coleoptera and family Tenebrionidae. This species is a serious secondary pest throughout the warmer parts of the world in food stores. Infestation is apparently by the appearance of adults on the surface of the grain: there is extensive damage to previously holed or broken grains, or grain damage to previously damaged by other pests. Damage is done by both larvae and adults.
Distribution
This pest is cosmopolitan in warmer countries.
Life History
The female lays small, cylindrical and white eggs scattered in the produce. The larvae are yellowish-white, about 6 mm long when fully grown. The head is pale brown, and the last segment of the abdomen has two upturned dark pointed structures. The larvae live and develop inside the grain till pupation. The damage to the stored grains is done by the larvae. The pupa is yellowish-white, later becoming brown, the dorsum hairy, and the tip of the abdomen having two spine-like processes. The adult is rather flat, oblong, reddish-brown in colour, and about 3-4 mm long. Each female is capable of laying 400-500 eggs and the adults are long-lived, under some circumstances living for a year or more. The life period from egg to adult is 35 days at 30 ˚C. Adults fly in large numbers in the late afternoon (Dennis, 1983).
Damage
Infestation is apparent by the appearance of adults on the surface of the grain; there is extensive damage to previously holed or broken grains, or grain damaged by other pests. Damage is done by both larvae and adults.
Control
The shelled grain should be thoroughly admixed with BHC (Benzene Haxa Chloride) dust or powder, if locally permitted. Fumigation should only be carried out by approved operators.
Melaleuca leucadendron
The M. leucadendron is commonly known as cajuput tree, punk, weeping paperbark, milk wood, white wood, paper bark tree, swamp tea tree, tea tree, white tea tree and brown tea tree (Bailey, 1958). It is classified in the kingdom Plantae, class Magnoliopsida, order Myrtales and family Myrtaceae.
M. leucadendron is a large tree, native to Southeast Asia, the Pacific Islands and Australia. It can be found in many countries such as Indonesia, Malaysia, Vietnam, the Philippines, Thailand, Cuba, Venezuela, Turkey, United States and Hawaii (Alonso et al., 1996; De Colmenares et al., 1998; Kitanov et al., 1992). The tree can be used for many purposes such as essential oil, medicinal properties, fuel wood, timber, charcoal and shelter belts (Brinkman and Vo, 1991).
The oil of M. leucadendron leaf derived by steam distillation (known as cajuput oil or tea tree oil) is green in color with a camphor odor and is used medicinally such as antiseptic, stomachic, stimulant, analgesic, antirhematic, expectotant, and for treatment of intestinal worms (Usher, 1974; Kitanov et al., 1992). This essential oil contains 1, 8 – cineole, nerolidol, alloromadendrene, viridiflorol and α – terpineol as the major components (De Colmenares et al., 1998; Pino et al., 2002).
Botanical Insecticides
Several higher plants produce secondary metabolites that are considered as defensive phytochemicals inhibiting herbivory and infestation by microorganisms (Green and Hedin, 1986; Klocke, 1989; Rosenthal and Berenbaum, 1991). In addition, plant allelochemicals act as repellents, growth regulators and antifeedants (Jermy, 1990). Therefore, these plants may provide new sources of natural pesticides and antifeedants which can be used to replace heavy insecticides applications (Grainge and Ahmed, 1988; Ananthakrishnan, 1992). Antifeedant or feeding deterrent is often highly specific in action, and advantages in integrated pest management (IPM) applications (Whitehead and Bowers, 1983).
The indiscriminate use of chemical pesticides has given rise to many serious problems, including genetic resistance of pest species, toxic residues, increasing costs of application, environment pollution, hazards from handling etc. (Ahmed et al., 1981; Khanam et al., 1990). The development of cross- and multi-resistance strains in many important insect species, resulting from the continuous use of chemical insecticides, has been reported from all over the world (Dyte, 1970; Pasalu and Bhatia, 1983; Dyte and Halliday, 1985; Irshad and Gillani, 1990; Zettler and Cuperus, 1990; Zettler, 1991).
For several years, many plant species are being investigated for their natural products to be used as pest control. For example, some active ingredients of seeds and leaves of the tropical neem tree, Azadirachta indica, especially the tetranortriterpenoid azadirachtin, influence the feeding behavior, metamorphosis, fecundity and numerous insect species belonging to various orders (Jacobson, 1989). Neem oil, obtained by cold-pressing seed, can be effective against soft-bodied insects and mites but is also useful in the management of phytopathogens (Isman et al., 1996).
The use of neem leaves in protecting stored grain and other commodities is as age-old practice in India. Pruthi (1937) and Pruthi and Singh (1950) reported the efficacy of neem leaves in protecting stored grain from insect attack. Jotwani and Sircar (1965) showed that the neem kernel was effective as a grain protectant against stored grain insect pests.Yadav (1983) found that neem kernel protects leguminous seeds from attack by Callosobruchus maculatus (F.) and C. chinensis (L.)
Pandey et al., (1976) observed that 1 to 3 parts of neem oil per 100 parts of seed effectively protects Bengal gram (Cicer arietinum) seeds from damage by the bruchid C. chinensis for at least 135 days, without any adverse effects of treatment on germination. Ketkar (1976) reported the efficacy of neem kernel powder and neem oil in controlling the grain pests, under existing godong (warehouse) conditions.
Apart from those natural products in current use such as pyrethrins, rotenone, nicotine, ryania, sabadilla and neem oil, several substances of plant origin have been identified as having toxic, repellent, antifeedant, and/or growth- and development inhibiting-potential on arthropod pests (Coats, 1994). The potential of essential oils to act as fumigants has been demonstrated for stored-product pests (Klingauf et al., 1983; El-Nahal, et al., 1989; Singh et al., 1989;, Shaaya et al., 1991; Rice and Coats 1994; Saraç and Tunç 1995). These results indicate that essential oils may be efficacious and safe replacements for conventional synthetic fumigants.
In Togo, Adhikary (1981) compared neem leaf and seed powder with conventional insecticides for protecting corn stored in sacks or unpeeled corn cobs in bins against S. zeamais, Tribolium spp. R. dominica and Cathartus spp.
As spices are commonly used in cooking in various parts of the world, they are generally considered to be relatively harmless to humans compared with non-edible plants. In addition, spices possess various insect control properties. For example, acetone and hexane extracts of peppercorn are toxic to several species of stored product insects (Su, 1984). Chopped garlic and ethyl acetate extracts of garlic were found to repel T. castaneum and S. zeamais (Ho et al., 1987). The essential oil of garlic has also been demonstrated to be insecticidal to T. castaneum and S. zeamais (Ho et al., 1996). The essential oil of several spices, such as anise (P. anisum) and peppermint (M. piperita) have fumigant toxicity to four major stored product pests, R. dominica, T. castaneum, S. oryzae and O. surinamensis (Ho et al., 1994) and star anise (Illicium verum Hook F) (Ho et al., 1995) showed insecticidal to T. castaneum and S. zeamais and suppressed progeny production. The main constituent of star anise, anethole, was also insecticidal to these two stored product insects and suppressed progeny production (Ho et al., 1997).
The addition of acetone extracts of black pepper and nutmeg seeds reduced the number of F1 progeny and prolonged the developmental period of S. zeamais (Haryadi and Rahayu, 2003). Himalayan cedarwood oil drilled from the wood chips of Cedrus deodara was found to possess its toxicity to the pulse beetle (Singh and Rao, 1985) and protection against rice weevil (Singh et al., 1989).
Materials and Methods
Insects
Two species of insects from Department of Agriculture, Ministry of Agriculture and Co-operative, Thailand will be used in this study: S. zeamais (maize weevil) and T. castaneum ( red flour beetles). S. zeamais will be reared on rice 12–13% moisture content while T. castaneum will be reared on rice bran. The cultures will be maintained in the laboratory at 29-32 ˚C and 70–80% RH.
Extraction of Essential Oils
Fresh leaves or fruits of selected plants will be collected from several places or bought from local market, where no pesticides are applied. The fresh leaves will be cut into small pieces and placed in 5 l extracting flask before steaming on the heating mantle for 6–8 h. Then, essential oil will be collected and kept in the refrigerator for further use.
Repellency Test
Petri dishes of 9 cm in diameter will be used to confine insects during experiment. Melaleuca essential oil will be diluted in ethanol to different concentrations (1%, 2%, 3% and 4%) and ethanol absolute will be used as control. Filter papers (9 cm diameter) will be cut in half. One ml of Melaleuca essential oil will be applied to one half of the filter paper as uniformly as possible with a micropipette. The other half (control) will be treated with 1 ml of ethanol absolute. Both treated half and control sides will then be air–dried to evaporate the solvent completely. Full disc will be carefully remade by attaching tested halves to control halves with sellotape. Precautions should be taken so that attachment will not prevent the free movement of insects from one half to another, but the distance between the filter-paper halves remained sufficient to prevent seepage of test samples from one half to another. Each remade filter paper will be placed in a petri dish with the seam oriented in one of four randomly selected different directions to avoid any insecticidal stimuli affecting the distribution of insects. Ten insects will be released at the center of each filter-paper disc and a cover will be placed on the petri dish. For each essential oil, five replicates will be used and the experiment will be repeated once. Counts of the insects present on each strip will be made after 1 h and at hourly interval up to the fifth hour.
Fumigant Toxicity
Filter papers (2 cm diameter) will be impregnated with aliquots of 25 µl of an appropriate concentration of Melaleuca essential oil. Ethanol absolute will be used as control. After evaporating the solvent or ethanol for 2 min in air, each filter paper will be placed on the underside of the screw cap of a glass vial (2.5 cm diameter, 5.5 cm height). The cap will be screwed tightly onto the vial containing ten adults of either species of insects. Each concentration and control will be replicated five times, and the experiment will be repeated once. After 24 h, the insects will be transferred to holding cages with culture medium in incubator for the determination of end-point LC50 and LC95 values.
Contact Toxicity
The filer paper impregnation method will be used to examine the contact toxicity. Filter papers (9 cm diameter) will be impregnated with aliquots of 0.5 ml of an appropriate concentration of different essential oils. Ethanol absolute will be used for the control in these experiments. The filter papers will be air dried for 1 h. A glass ring (5.4 cm diameter, 2.5 cm depth) will be placed on each filter paper before 20 insects will be confined to each glass ring. Sticky substance will be applied to the inner surface of each ring to prevent the insects from climbing onto the side of the ring. For adult S. zeamais and T. castaneum, five replicates will be set up for each essential oil concentration and control, and experiment will be repeated once and kept them till new generation.
Flour Disk Bioassay
Aliquots of 200 µl of a suspension of wheat flour in water (10 g in 50 ml) will be dropped onto a clean plastic placed in a tray. The disks will be left in the fume hood overnight to dry, after which they will be put in an oven at 60 ˚C for 24 h. Melaleuca essential oil will be diluted in ethanol to get different concentrations (2%, 4% and 6%) and ethanol absolute and no ethanol will be used as two controls. After evaporation of the solvent, the disks will be placed in glass vials (diameter 2.5 cm, height 5.5 cm). Ten group-weighed, unsexed adults will be added to each preweighed vial containing the disk. Five replicates will be prepared. After three days, the glass vials with flour disks and live insects will be weighed again, and mortality of insects, if any, will be recorded. In order to eliminate the decrease in weight of flour disks due to the evaporation of ethanol and Melaleuca essential oil, the weight of one and two flour disks treated with ethanol or various concentrations of Melaleuca essential oil will be recorded. After three days, the disks will be weighed again to determine the decrease in their weight. The weight of the flour disks used in the experiments will be corrected for any decrease in weight due to evaporation and drying. Nutritional indices will be calculated with some modifications: relative growth rate (RGR) = (A-B)/B x day-1, where A = weight of live insects on the third day (mg)/No. of live insects on the third day, B = original weight of insects (mg)/No. of insects; relative consumption rate (RCR) = D/B x day-1, where D = biomass ingested (mg)/No. of live insects on the third day; efficiency of conversion of ingested food (ECI)(%) = (RGR/RCR) x 100. For antifeedant action, the formula described by Isman et al. (1990) will be modified in calculating the feeding deterrence index (FDI)(%) = (C-T)/C x 100, where C = the consumption of control disks and T = the consumption of treated disks, as the control and treated disks will be placed in separate vials in no-choice tests.
Data Analysis
Data in all experiment will be analyzed by SAS program, and Least Significant Difference Test (LSD) will be analyzed to compare the treatment means (P <>Experimental Place and Duration
Department of Entomology, Faculty of Agriculture, Kasetsart University, Bangkok, Thailand (June - 2007 to Jan - 2009).
Study Supported
All of these studies are under financial support by the government of The Union of Myanmar under technical support by FAO for Myanmar Oil Crops Development Project.
Literature Cited
Abbot, W. S. 1925. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 18: 265-267.
Adhikary, S. 1981. Togo experience in moving from neem research to its practical application for plant protection. pp. 215-222. In H. Schmutterer., K.P.R. Ascher and H. Rembold, eds. Proc. 1st Int. Neem conf. (Rotach-Egern, Germany, 1980).
Ahmed, S. M., H. Chander and J. Pereira. 1981. Insecticidal potential and biological activity of Indian indigenous plants against Musca domestica. Int. Pest Control. 23: 170-175.
Aliaga, T. J and Feldheim, W. 1985. Hemmung der Keimbildung bei gegagerten Kartoffeln durch das ätherische older südamerikanischen Munapflanze (Minthostachys spp.) Ernährung 9: 254-256.
Alonso, O. S., M. D. C. Sanchez and A. Delgado. 1996. The oil extract Cajeput, a repellent and bio-insecticide against Andrector ruficornis. Pastosy Forrajes 19: 289-293.
Alonso-Anelot, M. E., J. L. Avila, L. D. Otero, F. Mora and B. Wolff. 1994. A new bioassay for testing plant extracts and pure compounds using red flour beetle Tribolium castaneum Herbst. J. Chem. Ecol. 20: 1161-1177.
Ananthakrisnan, T. N. 1992. Dimensions of Insect-Plant Interactions, Oxford and IBH Publishing, New Delhi.
Bailey, L. H. 1958. The standard cyclopedia of horticulture Macmillan press, New York.
Bell, E. A., L. E. Fellows and M. S. J. Simmonds. 1990. Natural products from plants for the control of insect pests. pp. 337-350. In E. Hodgson and R. J. Kuhr (eds.). Safer Insecticides. M. Dekkar, New York.
Breuer, M. and G. H. Schmidt. 1995. Influence of a short period treatment with Melia azedarach extract on food intake and growth of the larvae of Spodoptera frugiperdu (Smith) (Lepidoptera: Noctuidae). J. Pl. Dis. Prot. 102: 633-654.
Brinkman, W. J. and T. X. Vo. 1991. Melaleuca leucadendron, a useful and versatile tree for acid sulphate soils and some other poor environments. J. Int. Tree Crops 6: 261-274.
Coats, J. R. 1994. Risks from natural versus synthetis insecticides. Annu. Rev. Entomol. 39: 489-515.
De Colmenares, N. G., G. O. De Rodriguez, A. Prieto, O. Crescente and L. Cabrera. 1998. Phytoconstituents and antimicrobial activity of Melaleuca leucadendron leaf essential oil from Venezuela. Ciencia (Maracabo) 6: 123-128.
Dennis, S. H. 1983. Agricultural Insect Pests of the Tropics and Their Control (2nd eds.). Cambridge University Press, Cambridge, New York, 437 p.
Devlin, J. F and T. Zettle. 1999. Ecoagriculture: Initiative in Eastern and Sothern Africa. Weaver Press. Harare.
Dhirendra, M., N. Misra and D. Misra. 1989. Antifungal activity of cajeput oil against Aspergillus fumigatus (EIDAM) Wint. (NRRL 1979) and Fusarium moniliforme Sheldon (NRRL 6398). Indian Perf. 33; 151-155.
Dimock, M. B and J. A. A. Renwick. 1991. Oviposition by field populations of Pieris rapae (Lepidoptera: Pieridae) deterred by an extractant of a wild crucifer. Environ. Entomol. 20: 802-806.
Dubey, N. K., N Kishore, S. K. Singh and A. Dikshit. 1983. Antifungal properties of the volatile fraction of Melaleuca leucadendron. Trop. Agric. 60: 227-228.
Dyte, C. E. 1970. Insecticide resistance in stored-product insects with special reference to Tribolium castaneum. Trop. Stored Prod. Inf. 20: 13-15.
Dyte, C. E. and D. Halliday. 1985. Problems of development of resistance to phosphine by insect pests of stored grains. Bull. Org. Europe. Mediter. Prot. Plant. 15: 51-57.
El-Ibrashy, M. T. 1974. Sterilization of the Egyptian cotton leafworm Spodoptera littoralis (Boisd.) with a foliage extract of Podocarpus gracilior J. Appl. Entomol. 75: 107-109.
El-Nahal, A. K. M., G. H. Schimidt, and E. M. Risha. 1989. Vapours of Acorus calamus oil-a space treatment for stored-product insects. J. Stor. Prod. Res. 25: 211-216.
Farag, R. S., Z. Y. Daw, M.A.S. Mahassen and H. M. Saffaa. 1998. Biochemical and biological on some tea trees (Melaleuca spp.) essential oils. Adv. Food Sci. 20: 153-162.
Finney, D. J. 1971. Probit Analysis, University press, Cambridge, UK. 333 p.
Fitzjarrell, E. A. 1995. Method and formulation for elimination fleas on animals-USPTO Patent full-text and image database. United States Patent. 5,449,517, United States.
Grainge, N. and S. Ahmed. 1988. Handbook of Plants with Pest Control Properties. Wiley. New York.
Green, M. B and P. A. Hedin. 1986. Natural Rresistance of Plants to Pests: Role of Allelochemicals. ACS symposium Series. American Chemical Society, Washinton DC.
Haryadi, Y. and S. Rahayu. 2003. Study on the effects of mixture of acetone extracts of black pepper (Piper nigrum L.) and Nutmeg (Myristica fragrans Houtt) seeds on the development of Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). pp. 863-865. In P.F. Credland, D. M. Armitage, C.H. Bell. P. M. Cogan and E. Highley eds. Advances in Stored Product Protection, Proc. of the 8th Int. Working Conference on Stored Product Protection (2003). 22-26 July 2002. York, UK.
Hermawan, W., S. Kojiyama, R. Tsukuda, K. Fujisaki, A. Koboyashi and F. Nakasuji. 1994. Antifeedant and antioviposition activities of fraction of extract from a tropical plant Andrographis paniculata (Acanthaceae) against the diamondback moth, Plutella xylostella (Lepidoptera: Yponomeutidae). Appl. Entomol. Zool. 29: 533-538.
Herve, B. D. 1981. La munia como insecticida casero. Contribusion Regional Tecnica Apropriada, Bolivia, pp. 24-25.
Hiremath, I. G., Y. J. Ahn, and S.I. Kim. 1997. Insecticidal activity of Indian plant extracts against Nilaparvata lugens (Homoptera: Delphacidae). Appl. Entomol. Zool. 32: 159-166.
Ho, S. H., Y. Ma and H. T. W. Tan. 1987. Repellency of some plant extracts to the stored products beetles, Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch. Proc. Symp. Pest Management Stored Food and Feed, SEMEO BIOTROP, Bogor, Indonesia, September, 1995.
Ho, S. H., L. P. L. Cheng, K. Y. Sim and H. T. W. Tan. 1994. Potential of clove (Syzygium aromaticum (L.) Merr. and Perry as a grain protectant against Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch. Postharvest Biol. Tech. 4: 179-183.
Ho, S. H., Y. Ma and K. Y. Sim. 1995. Star anise, Illicium verum Hook f., as a potential grain protectant against Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch. Postharvest Biol Tech. 6: 341-347.
Ho, S. H., L. Koh, Y. Ma, Y. Huang and K. Y. Sim. 1996. The oil of garlic, Allium sativum L. (Amaryllidaceae), as a potential grain protectant against Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch. J. Stor. Prod. Res. 34: 11-17.
Ho, S.H., Y. Ma and Y. Huang. 1997. Anethole, a potential insecticide from Illicium verum Hook f., against two stored product insects. Int. Pest Control. 39: 50-51.
Huang, Y and S. H. Ho. 1998. Toxicity and antifeedant activities of cinnamaldehyde against the grain storage insects, Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch. J. Stored Prod. Res. 34: 11-17.
Huang, Y., J. M. W. L. Tan, R. M. Kini and S. H. Ho. 1997. toxic and antifeedant action of nutmeg oil against Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch. J. Stored Prod. Res. 33: 289-298.
Irshad, M. 1989. Botanical insecticides Past, present and future. pp. 1-10. In Insecticides of Plant Origin. J. T. Arnason., B. J. R. Philogène and P. Morand (eds.). American Chemical Society Symposium Series No. 387, Washinton DC. American Chemical Society.
Irshad, M. and W. A. Gillani. 1990. Resistance in Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) against malathion, Pakistan J. Zool. 22: 257-262.
Isman, M. B., O. Koul, A. Luczynski and J. Kaminski. 1990. Insecticidal and antifeedant bioactivities of neem oils and their relationship to azadirachtin content. J. Agric. Food Chem. 38: 1406-1411.
Isman, M. B., H. Matsuura, S. MacKinnon, T. Durst, G. H. N. Towers and J. T. Arnason. 1996. Phytochemistry of the Meliaceae. So many terpenoids, so few insecticides. pp. 155-178. In Phytochemical Diversity and Redundacy. J. T. Romeo., J. A. Saunders and P. Barbosa (eds.) New York: Plenum.
Jacobson, M. 1989. Botanical pesticides: past, present and future. In Insecticide of Plant Origin. American Chemical Society, Washington DC.
Jermy, T. 1990. Prospects of antifeedant approach to pest control – a critical review. J. Chem. Ecol. 16: 3151-3166.
Jilani. G., R. C. Sazena and B. P. Rueda. 1988. Repellent and growth inhibiting effects of turmeric oil, sweetflag oil, neem oil and “Margosan-O” on red flour beetle (Coleoptera: Tenebrionidae). J. Econ. Entomol. 81: 1226-1230.
Jotwani, M. G. and P. Sircar. 1965. Neem seed as a protectant against stored grain pests infesting wheat seed. Indian J. Entomol. 27: 161-164.
Keita, S. M., C. Vincent, J. P. Schmit, S. Ramaswamy and A. Belanger. 2000. Effect of various essential oils on Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). J. Stor. Prod. Res. 36: 355-364.
Ketkar, C. M. 1976. Final Technical Report ‘Utilization of Neem (Azadirachta indica A. Juss) and its Byproducts’. Directorate of Non-edible Oils and Soap Industry, Khada and Village Industries Commision, Bombay, pp. 150-152.
Khan, Z. R. and R. C. Saxena. 1986. Effect of steam distillate extracts of resistant and susceptible rice cultivars in behavior of Sogatella furcifera (Homoptera: Delphacidae). J. Econ. Entomol. 79: 928-935.
Khanam, L. A. M., D. Talukder, A. R. Khan and S. M. Rahman. 1990. Insecticidal properties of Royna, Aphanamixis polysachya Wall. (Paker) (Meliaceae) against Tribolium confusum Duval. J. Asiat. Soc. Bangladesh Sci. 16: 71-74.
Kitanov, G. M., T. V. Dam, I. Assenov and T. Dam. 1992. Flavonols from Melaleuca leucadendron leaves, Fitoterapia. 63: 379-380.
Klepzig, K. D and F. Schlyter. 1999. Laboratory evaluation of plant derives antifeedants against the pine weevil Hylobious abictis (Coleoptera: Curculionidae). J. Econ. Entomol. 92: 644-650.
Klingauf, F., H. J. Bestman, O. Vostrovsky and K. Michaelis. 1983. Wirkung von ätherischen Ölen auf Schadinsekten. Mitteilungen der Deuschen Gesellschaft für Allgemeine und Angewandte Entomologie. 4: 123-126.
Klocke, J. 1989. Economic and medicinal plant research. In H. Wagner, J. Hikino and N. R. Farnsworth, eds. Plant compounds as models for insect control agents. Academic Press, New York.
Madrid, F.J., N.D.G. White and S.R. Loschiavo. 1990. Insects in stored cereals and their association with farming practices in Southern Manitoba. Can. Entomolo. 122: 515–523.
Menendez, J. M., M. D. C. Berrios and R. Quert. 1992. Preliminary study on the repellent effect of the essential oils of three species of the Myrtaceae on Wasmannia auropunctata. Revista Baracoa. 22: 47-50.
Metcalf, C.L., W. P. Flint and R. L. Metcalf. 1992. Destructive and useful insects. Mcgraw-Hill, New York.
Muthukrishnan, J. and E. Pushpalatha. 2001. Effects of plant extracts on fecundity and fertility of mosquitoes. J. Appl. Entomol. 125: 31-35.
Nawawi, A., N. Nakamura, M. Hattori, M. Kurokawa and K. Shiraki. 1999. Inhibitory effects of Indonesian medicinal plants on the infection of Herpes Simplex Virus Type I. Phytother. 13: 37-41.
Pandey, N. D., R. S. Shiva and G. C. Tiwari. 1976. Use of some plant powders, oils and extracts as protectant against the pulse beetle, Callosobruchus chinensis L. Indian J. Entomol. 38: 110-113.
Papachristos, D. P and D. C. Stamopoulos. 2002. Toxicity of vapors of three essential oils to the immature stages of Acanthoscelides obtectus (Say) (coleopteran: Bruchidae). J. Stored Prod. Pes. 38: 365-373.
Pasalu, I. C and S. K. Bhatia. 1983. Inheritance of resistance to malathion in Tribolium castaneum (Herbst). Proc. Indian Acad. Sci. (Anim. Sci.). 92: 409-414.
Perkins, J. H. 1985. Naturally occurring pesticides and the pesticide crisis. 1945 to 1980. pp. 297-325. In N. B. Mandava, ed. Handbook of Natural Pesticides: Methods. Vol. 1: Theory, Practice and Detection. CRC. Boca. FL.
Pino, J., A. Bello, J. Urquiola, J. Aguero and R. Marbot. 2002. Chemical composition of cajuput oil (Melaleuca leucadendron L.) from Cuba. J. Esocent. Oil Res. 14: 10-11.
Pruthi, N. S. 1937. Report of the Imperial Entomologist, Science. Rept. Agric. 1st. New Delhi, India. 1935-36.
Pruthi, N. S and M. Singh. 1950. Pests of stored grain and their control. Indian J. Agric. Sci. 18 (4): 52 (special No. Vol. 18, 1948).
Rembold, H. 1988. Isomeric azadirachtins and their mode of action. pp. 47-67. In M. Jacobson, ed. Phytochemical Pesticides. Vol.1. The Neem Tree. CRC, Boca Raton.
Rice, P. J and J. R. Coats. 1994. Insecticidal properties of several monoterpenoids to the housefly (Diptera: Muscidae), red flour beetle (Coleoptera: Tenebrionidae) and southern corn rootworm (Coleoptera: Chrysomelidae). J. Econ. Entomol. 87: 1172-1179.
Rodriguez-Saona, C. R. and J. T. Trumble. 1999. Effect of ovocardofurans on larval survival, growth and food preference of the generalist herbivore, Spodoptera exigua. Entomol. Expt. Appl. 90: 131-140.
Rosenthal, G. A. and M. R. Berenbaum. 1950. Pests of stored grain and their control. Indian J. Agric. Sci. 18 (4): 52 (special Number Vol: 18, 1948)
Sadek, M. M. 1997. Antifeedant and larvicidal effects of Eichornia crassipes leaves on the cotton leafworm Spodoptera littoralis (Boisd.). J. Egypt.Ger. Soc. Zool. 24 (E): 209-232.
Saraç, D. and I. Tunç. 1995. Toxicity and repellency of essential oils to stored-product insects. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz. 102: 429-434.
Schmutterer, H. 1990. Properties and potential of natural pesticides from the neem tree. Ann. Rev. Entomol. 35: 271-297.
Shaaya, E., M. Kostjukovski, J. Eilberg and C. Sukprakarn. 1997. Plant oils as fumigants and contact insecticides for the control of stored-product insects, J. Stor. Prod. Res. 33: 7-15.
Shaaya, E., U. Ravid, N. Paster, B. Juven and U. Zisman. 1991. Fumigant toxicity of essential oils against four major stored-product insects. J. Chem. Ecol. 17: 499-504.
Sharaby, A. 1988. Evaluation of some Myrtaceae plant leaves as protectant against the infestation by Sitophilus oryzae L. and Sitophilus granarius L. Insect Sci. Appl. 9: 465-468.
Singh, B. B and R. K. Upadhyay. 1993. Essential oils: a potent source of natural pesticides. J. Sci. Indus. Rs. 52: 676-683.
Sighamony, S., I. Anees, T. Chandrakala and J. Osmani. 1986. Efficiency of certain indigenous plant products as grain protectants against Sitophilus oryzae (L.) and Rhyzopertha dominica (F.) J. Stored Prod. Res. 22: 21-23.
Simpson. B. B. 1995. Spices, herbs and perfumes. pp. 278-301. In B. B. Simpson and M. C. Ogorzaly, eds. York.
Singh, D. and S. M. Rao. 1985. Toxicity of cedarwood oil against pulse beetle, Callosobruchus chinensis Linn. Indian Perf. 29: 201-204.
Singh, D., M. S. Siddiqui and S. sharma. 1989. Reproduction retardant and fumigant properties in essential oils against rice weevil (Coleoptera: Curculionidae) in stored wheat. J. Econ. Entomol. 82: 727-733.
Spurgeom, D. 1977. Hidden Harvest: A system approach to post-harvest technology. PAG Bull. 7: 45-48.
Su, H. C. F. 1984.Comparative toxicity of three peppercorn extracts to four species of stored-product insects under laboratory conditions. J. Georgia Entomol. Soc. 19: 190-199.
Tripathi, A.K. V. Prajapati, K. K. Aggarwal and S. Kumar. 2001. Toxicity, Feeding deterrence and effect of activity of 1,8 cineole from Artemisia annua on progeny production of Tribolium castaneum (Coleoptera: Tenebrionidae). J. Econ. Entomol. 94: 979–983.
Tunc, I. and S. Sahinkaya. 1998. Sensitivity of two greenhouse pests of vapours of essential oils. Entomol. Exp. Appl. 86: 183-187.
Usher, B. 1974. Dictionary of Plants Used by Man. Constable and Co., London.
Ware, G. W. 1986. Fundamentals of pesticides. Thompson Publishing Frenco. Ca.
Warthen, J. D., Jr. 1979. Azadirachta indica: a source of insect feeding inhibitors and growth regulators. U.S. Dept. Agric. Rev. Mon. ARM-NE-4.
Weiss, E. A. 1997. Litsea cubeta. In Essential oil crops. CAB International, Wallingford, Oxon Ox 10 820, UK. pp. 207.
Wheeler, D. A. and M.B. Isman. 2000. Effect of Trichilia American extract on feeding behavior of Asian armyworm, Spodoptera litura, J. Chem. Ecol. 26: 2791-2800.
Whitehead, D. L. and W. S. Bowers. 1983. Natural products for innovative pest management. Pergamon. Oxford.
Yadav, T. D. 1983. Studies on the Insecticidal Treatment Against Bruchids Callosobruchus maculatus (Fabr.) and C. chinensis L. Damaging Stored Leguminous Seeds Ph. D. thesis, Ara University, Agra (U.P), India.
Yidirim, E., H. Ozbek and I. Aslan. 2001. Pests of stored product, Ataturk University Agricultural Faculty Press (2001). No.191. pp. 117
Zettler, J. L. and G. W. Cuperus. 1990. Pesticide resistance in Tribolium castaneum (Coleoptera: Tenebrionidae) and Rhizopertha domineca (Coleoptera: Bostrichidae) in wheat. J. Entomol. 83: 1677-1681.
1995.
Zettler, J. L. 1991. Pesticide resistance in Tribolium confusum (Coleoptera: Tenebrionidae) from flour mills the USA. J. Econ. Entomol. 84: 763-767.
Zhao, B., G. G Grant, D. Langevin, and L. Macdonald. 1998. Deterring and inhibiting effects of quinizidine alkaloids in spruce budworm (Lepidoptera: Tortricidae) oviposition. Environ. Entomol. 27: 984-992.




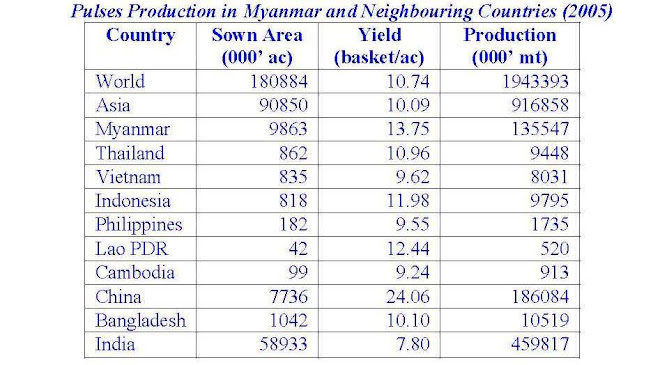
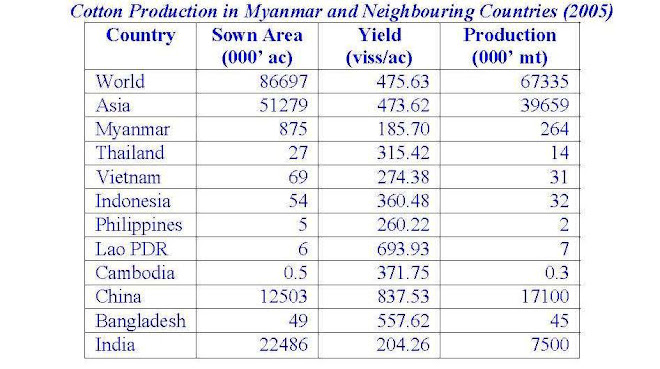+of+Paddy1.jpg)
